The battery charging process is reverse process to battery discharging. In discharging process, the active electrons become lesser and the internal resistance becomes high causing a low output this happen due to chemical reaction. To reverse this action, send a DC current through the battery in the opposite direction to that of the discharge, this process is called as charging. The battery can be charge by a battery charger.
Table of Contents
ToggleBattery charging by chargers:
When the chemical reaction in a battery has ended, the battery is said to be discharged and cannot produce the rated flow of electrons. This battery can be recharge, by passing direct current (DC) from an outside source. The direction of current opposite to that in which it flowed out of the battery in discharging process.
For charging a battery, the negative lead of the charger must connect to the negative terminal of the battery and the positive lead of the charger to the positive terminal of the battery.
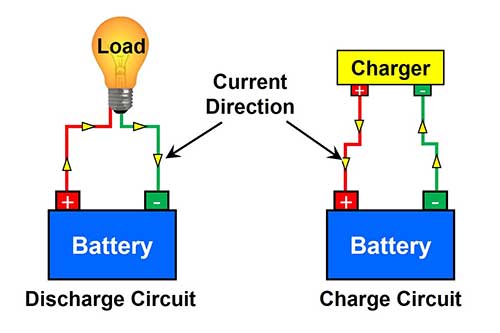
Figure shows the direction of current and terminal connection of battery in charging and discharging mode.
If this connection is revers by mistake, it will produce a short circuit and may damage both the charger as well as the battery. A simple variable voltage DC power supply works well as a battery charger.
Battery Charging current:
When charging any battery, it is important to set the charging current value as recommended by the manufacturer. When the battery and charger are at the same voltage value, no current will flows through circuit. Therefore the charger voltage is set to a value higher than that of the battery to produce a flow of current.
Battery charging condition can be observed by the following points.
1) Specific gravity of the electrolyte.
2) Ampere hour capacity of each cell.
3) Voltage of each cell of the battery.
Important point about Electrolyte:
The electrolyte used in a battery is dilute sulphuric acid having a specific gravity between 1.21 and 1.3.
But the specific gravity of the acid available in the market is usually 1.835.
To reduce specific gravity of the acid, it gently poured into distilled water.
In this way, the acid is diluted up to a specific gravity of 1.4, it to be filled up in the battery, then it is further diluted up to a specific gravity of 1.25.
MCQ Questions PDF of Module 7: Cells and Batteries
- Primary cells and secondary cells.
- Grouping of cells.
- Battery charging method – Battery charger.
- Installation, care and maintenance of batteries.
- Solar cells.
Download MCQ Questions PDF of Cells and Batteries
This MCQ Question Is Taken From Bharat Skill Website Published By NIMI.
Objective questions on the above topics explain in the video on YouTube.